Discover The Intriguing Melting Point Of Graphite
The melting point of graphite is the temperature at which it transitions from a solid to a liquid state. It is an important property for understanding the behavior of graphite in various applications.
The melting point of graphite is influenced by several factors, including the pressure, the presence of impurities, and the crystal structure of the graphite. Under normal atmospheric pressure, the melting point of graphite is approximately 3,652 degrees Celsius (6,606 degrees Fahrenheit).
The high melting point of graphite makes it an ideal material for high-temperature applications, such as crucibles, electrodes, and heat shields. It is also used in the production of carbon fibers, which are used in a variety of products, including aerospace composites, sporting goods, and medical devices.
Read also:Intriguing Discovery Doraemon Body Found In Sea A Deep Dive Read also:Age Insights Karoline Leavitt Husbands Age And Personal Life Read also:The Intriguing Life Of Lola Young Mixed Race A Celebration Of Diversity And Talent
melting point for graphite
The melting point of graphite is a critical property that determines its behavior in various applications. Here are eight key aspects related to the melting point of graphite:
- High temperature: The melting point of graphite is exceptionally high, making it suitable for high-temperature applications.
- Pressure dependent: The melting point of graphite can vary depending on the pressure applied.
- Impurity influence: The presence of impurities can affect the melting point of graphite.
- Crystal structure: The crystal structure of graphite also plays a role in determining its melting point.
- Crucible material: Graphite's high melting point makes it an ideal material for crucibles used in high-temperature processes.
- Electrode applications: Graphite electrodes are commonly used in various industrial processes due to their high melting point.
- Heat shields: Graphite's ability to withstand high temperatures makes it useful in heat shield applications.
- Carbon fibers: The melting point of graphite is crucial for the production of carbon fibers, which are used in various high-performance materials.
In summary, the melting point of graphite is a key property that determines its suitability for various high-temperature applications. Understanding the factors that influence the melting point of graphite is essential for optimizing its performance in these applications.
1. High temperature
The melting point of graphite is a crucial property that determines its suitability for various high-temperature applications. Graphite's exceptionally high melting point, approximately 3,652 degrees Celsius (6,606 degrees Fahrenheit) under normal atmospheric pressure, makes it an ideal material for applications that require resistance to extreme heat.
One of the key applications of graphite's high melting point is in the production of crucibles. Crucibles are containers used to hold and melt materials at high temperatures. Graphite crucibles are commonly utilized in industries such as metallurgy, glass manufacturing, and chemical processing, where the ability to withstand extreme temperatures is essential.
Another important application of graphite's high melting point is in the manufacturing of electrodes. Electrodes are conductors used in various industrial processes, including electric arc furnaces and electrolysis cells. Graphite electrodes are highly sought after due to their ability to withstand high temperatures and their excellent electrical conductivity.
Furthermore, graphite's high melting point makes it a valuable material for heat shields. Heat shields are designed to protect sensitive components or structures from extreme heat. Graphite's ability to withstand high temperatures without melting or decomposing makes it an ideal material for applications such as spacecraft heat shields and industrial furnace linings.
Read also:The Ultimate Guide To Winnie The Pooh Quotations Read also:Meet Emma Culligan A Closer Look At Her Remarkable Journey Read also:Jameliz Benitez Smith Nude
In summary, the exceptionally high melting point of graphite is a critical property that enables its use in various high-temperature applications, including crucibles, electrodes, and heat shields. Understanding the connection between the high melting point and the suitability of graphite for these applications is essential for optimizing its performance and ensuring the successful implementation of high-temperature processes.
2. Pressure dependent
The melting point of a substance is typically defined as the temperature at which it transitions from a solid to a liquid state at a given pressure. However, for some materials like graphite, the melting point can also be influenced by the pressure applied.
In the case of graphite, the melting point increases with increasing pressure. This means that graphite requires a higher temperature to melt when it is subjected to higher pressure. This phenomenon can be attributed to the changes in the interatomic bonding and crystal structure of graphite under pressure.
Understanding the pressure dependence of graphite's melting point is crucial for various applications. For instance, in high-pressure environments such as the Earth's mantle, graphite's melting point can be significantly elevated, affecting geological processes and the formation of diamond.
Furthermore, this pressure dependence has practical implications in industrial processes. For example, in the production of synthetic diamonds, high pressure and temperature conditions are employed to transform graphite into diamonds. Controlling the pressure and temperature parameters is essential to achieve the desired phase transition.
In summary, the pressure dependence of graphite's melting point is a significant property that influences its behavior under varying pressure conditions. Understanding this relationship is vital for optimizing graphite's performance in high-pressure applications and advancing our knowledge of materials science.
3. Impurity influence
The melting point of graphite, the temperature at which it transitions from a solid to a liquid state, is a critical property that determines its behavior in various applications. However, the presence of impurities can significantly influence the melting point of graphite.
Impurities in graphite can act as nucleation sites for the formation of new crystals. This can lead to a decrease in the melting point of graphite, as the presence of these nucleation sites allows the graphite to melt at a lower temperature than it would in a pure state. The extent to which impurities affect the melting point of graphite depends on the type and concentration of the impurities present.
Understanding the influence of impurities on the melting point of graphite is important for several reasons. For instance, in the production of high-purity graphite for electronic applications, it is crucial to minimize the presence of impurities to achieve the desired melting point and electrical properties. Additionally, in geological processes, the presence of impurities in graphite can affect the formation and properties of natural graphite deposits.
In summary, the presence of impurities can significantly influence the melting point of graphite, affecting its behavior in various applications. Understanding this relationship is essential for optimizing graphite's performance and tailoring its properties for specific applications.
4. Crystal structure
The crystal structure of graphite refers to the arrangement of carbon atoms within the graphite lattice. The unique layered structure of graphite, composed of stacked graphene sheets, significantly influences its melting point.
- Strong In-Plane Bonding: Within each graphene sheet, the carbon atoms are covalently bonded in a hexagonal lattice, resulting in strong in-plane bonding. This strong bonding contributes to the high melting point of graphite, as a significant amount of energy is required to overcome these bonds and disrupt the sheet structure.
- Weak Interlayer Bonding: In contrast to the strong in-plane bonding, the bonding between adjacent graphene sheets is relatively weak, primarily due to van der Waals forces. This weak interlayer bonding allows the graphene sheets to slide past each other, contributing to graphite's lubricating properties and influencing its melting behavior.
- Anisotropic Melting: The anisotropic nature of graphite's crystal structure leads to anisotropic melting behavior. Graphite melts more easily along the graphene planes (basal planes) than perpendicular to them. This anisotropy is attributed to the differences in bonding strength between the in-plane and out-of-plane directions.
- Influence of Defects: The presence of defects and impurities in the graphite crystal structure can affect its melting point. Defects disrupt the regular arrangement of carbon atoms, introducing local variations in bonding strength. These defects can act as nucleation sites for melting, potentially lowering the overall melting point of the graphite.
In summary, the crystal structure of graphite, characterized by strong in-plane bonding, weak interlayer bonding, anisotropic melting, and the influence of defects, plays a crucial role in determining its melting point. Understanding this relationship is essential for tailoring graphite's properties and optimizing its performance in various applications.
5. Crucible material
The high melting point of graphite, approximately 3,652 degrees Celsius (6,606 degrees Fahrenheit), makes it an ideal material for crucibles used in high-temperature processes. Crucibles are containers designed to withstand extreme temperatures and are commonly used in various industries, including metallurgy, glass manufacturing, and chemical processing.
The ability of graphite to withstand high temperatures without melting or decomposing makes it a suitable material for crucibles. Graphite crucibles can handle molten metals, salts, and other materials at elevated temperatures, providing a stable and reliable environment for various industrial processes.
For instance, in the production of steel, graphite crucibles are used to melt and refine the molten metal. The high melting point of graphite ensures that the crucible can withstand the extreme temperatures required for steel production without compromising its structural integrity.
Furthermore, graphite crucibles are commonly employed in the manufacturing of glass. The high melting point of graphite allows it to withstand the temperatures necessary to melt and shape the molten glass, contributing to the production of high-quality glass products.
In summary, the high melting point of graphite is a crucial property that makes it an ideal material for crucibles used in high-temperature processes. Understanding this connection is essential for optimizing the performance of crucibles and ensuring the successful implementation of various industrial processes that rely on high-temperature applications.
6. Electrode applications
The high melting point of graphite, approximately 3,652 degrees Celsius (6,606 degrees Fahrenheit), makes it an ideal material for electrodes used in various industrial processes. Electrodes are conductors that play a crucial role in electrochemical reactions and high-temperature applications.
- Electrical Conductivity: Graphite's high electrical conductivity makes it an effective material for electrodes. It can efficiently conduct electricity, allowing for the passage of current in electrochemical cells and electrical circuits.
- Resistance to High Temperatures: The high melting point of graphite ensures that it can withstand the extreme temperatures encountered in industrial processes. This property prevents the electrodes from melting or degrading, even at elevated temperatures.
- Chemical Stability: Graphite is chemically inert and resistant to corrosion, making it suitable for use in harsh environments. It can withstand exposure to various chemicals and molten materials without undergoing significant degradation.
- Machinability: Graphite is a relatively soft material that can be easily machined into complex shapes and sizes. This allows for the fabrication of electrodes with specific dimensions and geometries to meet the requirements of different applications.
In summary, the high melting point of graphite, coupled with its electrical conductivity, resistance to high temperatures, chemical stability, and machinability, makes it an ideal material for electrodes used in various industrial processes. Understanding this connection is essential for optimizing the performance of electrodes and ensuring the successful implementation of these processes.
7. Heat shields
The high melting point of graphite is directly connected to its ability to withstand high temperatures, making it a suitable material for heat shields. Heat shields are designed to protect sensitive components or structures from extreme heat, such as in spacecraft or industrial furnaces.
- Exceptional Thermal Resistance: Graphite's high melting point provides exceptional thermal resistance, enabling it to withstand extreme temperatures without melting or decomposing. This property makes it an ideal material for heat shields, as it can effectively protect underlying components from heat damage.
- Ablative Properties: In addition to its high melting point, graphite exhibits ablative properties. When exposed to high heat, a thin layer of graphite sublimates, absorbing heat and creating a protective gas layer that further shields the underlying structure.
- Lightweight and Durable: Graphite is a relatively lightweight material, making it suitable for applications where weight is a concern, such as in aerospace. Despite its lightweight nature, graphite is highly durable and can withstand the harsh conditions encountered in heat shield applications.
In summary, the high melting point of graphite, coupled with its exceptional thermal resistance, ablative properties, and lightweight durability, makes it an ideal material for heat shields. Understanding this connection is essential for optimizing the design and performance of heat shields in various applications.
8. Carbon fibers
The exceptional melting point of graphite, approximately 3,652 degrees Celsius (6,606 degrees Fahrenheit), plays a critical role in the production of carbon fibers. Carbon fibers are composed of thin, elongated strands of carbon atoms arranged in a highly ordered structure. They possess remarkable properties such as high strength, low weight, and excellent thermal and electrical conductivity.
The production of carbon fibers involves a process called chemical vapor deposition (CVD). In this process, a hydrocarbon gas, such as methane or propane, is passed over a heated substrate made of graphite. The high melting point of graphite is crucial at this stage as it enables the substrate to withstand the extremely high temperatures required for the CVD process, typically ranging from 900 to 1,300 degrees Celsius (1,652 to 2,372 degrees Fahrenheit).
During the CVD process, the hydrocarbon gas decomposes on the heated graphite substrate, releasing carbon atoms. These carbon atoms then rearrange themselves into the highly ordered structure of carbon fibers. The high melting point of graphite ensures that the substrate remains stable and does not melt or decompose, which is essential for the successful growth of high-quality carbon fibers.
The resulting carbon fibers are characterized by exceptional properties, making them ideal for use in various high-performance materials. They are widely employed in aerospace applications, such as aircraft and spacecraft components, due to their high strength-to-weight ratio and ability to withstand extreme temperatures. Additionally, carbon fibers are used in automotive parts, sporting goods, and medical devices, among other applications.
In summary, the high melting point of graphite is a critical factor in the production of carbon fibers. It enables the use of graphite as a substrate in the CVD process, ensuring the successful growth of high-quality carbon fibers with exceptional properties. Understanding this connection is essential for optimizing the production of carbon fibers and harnessing their remarkable qualities in a wide range of high-performance applications.
FAQs on Melting Point for Graphite
This section addresses frequently asked questions and misconceptions surrounding the melting point for graphite:
Question 1: What is the significance of the melting point for graphite?
Answer: The melting point of graphite is a crucial property that determines its behavior and suitability in high-temperature applications. It influences factors such as thermal stability, material strength, and resistance to deformation under extreme heat.
Question 2: How does the melting point of graphite compare to other materials?
Answer: Graphite has a remarkably high melting point of approximately 3,652 degrees Celsius (6,606 degrees Fahrenheit). This is significantly higher than most common metals and alloys, making it an ideal choice for applications requiring exceptional thermal resistance.
Question 3: What factors can affect the melting point of graphite?
Answer: The melting point of graphite can be influenced by various factors, including pressure, the presence of impurities, and the specific crystal structure of the graphite.
Question 4: Are there any practical applications that utilize the high melting point of graphite?
Answer: Yes, graphite's high melting point makes it suitable for various applications, such as crucibles for melting metals, electrodes in high-temperature processes, heat shields for spacecraft and industrial furnaces, and as a key component in the production of carbon fibers.
Question 5: How is the melting point of graphite measured?
Answer: The melting point of graphite can be measured using specialized techniques such as differential thermal analysis (DTA) or thermogravimetric analysis (TGA). These techniques involve heating a sample of graphite while monitoring its thermal behavior to determine the temperature at which it transitions from a solid to a liquid state.
Question 6: What are the implications of the melting point for graphite in industrial processes?
Answer: Understanding the melting point of graphite is crucial for optimizing industrial processes that involve extreme temperatures. It helps determine the suitability of graphite components, ensures their structural integrity, and prevents premature failure or degradation due to excessive heat.
In summary, the melting point for graphite is a critical property that governs its behavior under high-temperature conditions. It plays a vital role in various industrial applications and scientific research, and a comprehensive understanding of this property is essential for optimizing performance and ensuring successful outcomes.
Please note that if you have any further inquiries, you can consult scientific literature, technical data sheets, or reputable online sources for more in-depth information on the melting point for graphite and its implications.
Tips for Understanding and Utilizing the Melting Point for Graphite
Effectively navigating the technical aspects of graphite's melting point requires a strategic approach. Here are some valuable tips to guide you:
Tip 1: Grasp the Significance of Melting Point:
Recognize the melting point as a defining characteristic that dictates graphite's behavior under extreme heat. Comprehending its influence on properties like thermal stability and material strength is paramount.
Tip 2: Understand Influencing Factors:
Be aware of the variables that can impact the melting point of graphite. Factors such as pressure, the presence of impurities, and the specific crystal structure all play a role. Understanding these influences empowers informed decision-making.
Tip 3: Explore Practical Applications:
Harness the knowledge of graphite's high melting point to identify suitable applications. Its exceptional thermal resistance makes it ideal for components like crucibles, electrodes, heat shields, and carbon fiber production.
Tip 4: Utilize Measurement Techniques:
Employ specialized techniques like differential thermal analysis (DTA) or thermogravimetric analysis (TGA) to accurately measure the melting point of graphite. These methods provide reliable data for optimizing industrial processes.
Tip 5: Consider Industrial Implications:
Incorporate the understanding of graphite's melting point into industrial processes involving high temperatures. This knowledge ensures the proper selection of graphite components, safeguards their structural integrity, and prevents premature failure.
Tip 6: Consult Credible Sources:
Refer to scientific literature, technical data sheets, and reputable online resources for further insights into the melting point of graphite. Continuous learning and staying abreast of the latest research will enhance your expertise.
By implementing these tips, you can effectively comprehend and utilize the melting point for graphite in your research or industry endeavors. It empowers you to optimize performance, ensure successful outcomes, and push the boundaries of innovation.
Conclusion
The melting point for graphite is a critical property that governs its behavior under high-temperature conditions. Understanding this property is essential for optimizing the performance of graphite components, ensuring their structural integrity, and preventing premature failure in industrial processes.
The high melting point of graphite makes it an ideal material for applications such as crucibles, electrodes, heat shields, and the production of carbon fibers. By leveraging the exceptional thermal resistance of graphite, industries can achieve greater efficiency, enhance product quality, and push the boundaries of innovation.
Master The Art Of Photography With SnapGod: The Ultimate Guide
Spotlight On Sophie Rain: Unveiling The Rising Star
Master Your Podcasts: Unleash The Power Of Voice Changers
- Maltese Shih Tzu A Delightful And Charming Companion
- Farmington Nm A Comprehensive Guide To Its Rich History And Vibrant Culture
- Chinese Year Of The S 1965 Myths Facts And Cultural Significance

Understanding the Graphite Melting Point East Carbon
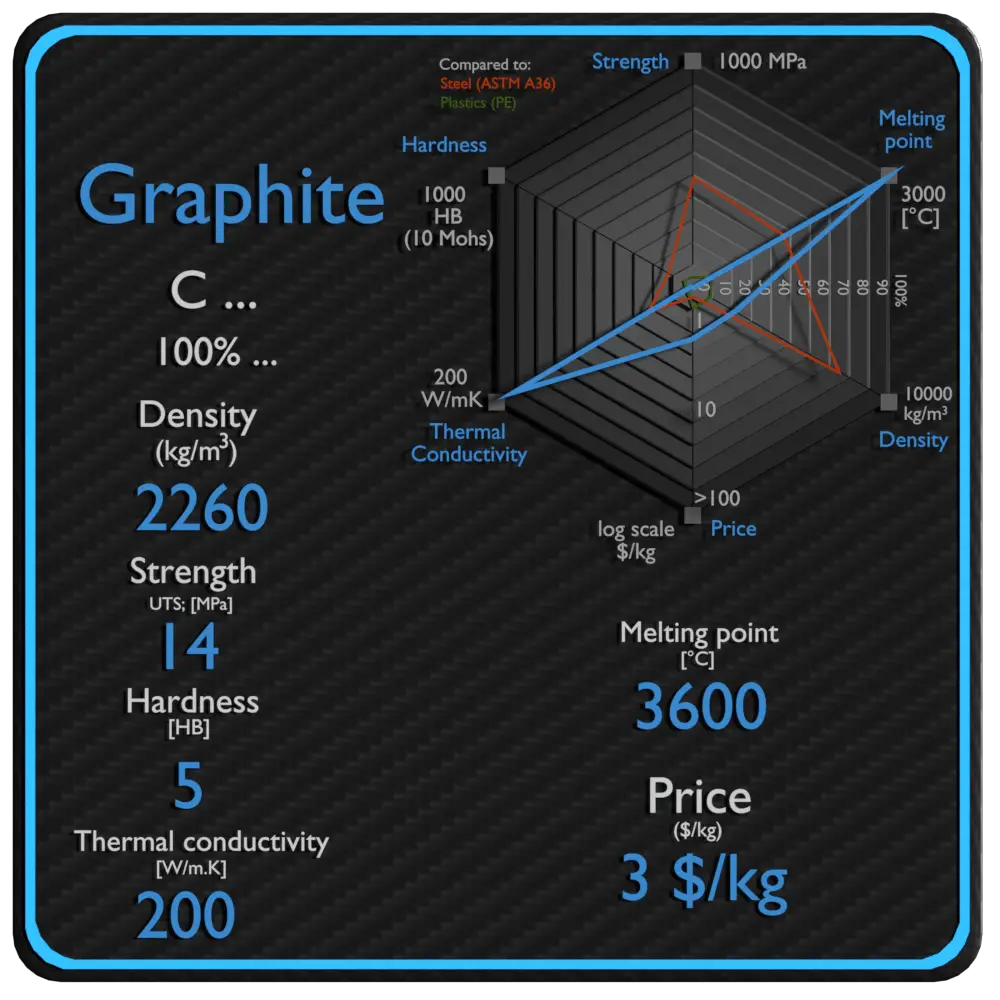
Graphite Density, Strength, Hardness, Melting Point

Graphite Electrode